What Is Acid–Alkaline Balance
The substances the body uses for building and functioning are quite numerous: there are approximately twenty amino acids, several dozen sugars and fatty acids, approximately forty vitamins, and one hundred or so minerals and trace elements. Each of these substances plays one or several specific roles in the body.
Despite the extreme diversity of these substances, it is possible to classify them in two major groups: basic (or alkaline) substances and acid substances. These two different groups of substances have opposing but complementary characteristics. To be healthy, the body needs both. When alkalines and acids are present in equal quantities, the acid–alkaline balance is achieved.
WHAT IS AN ACID?
If you have ever bitten into a lemon or eaten rhubarb you know the most obvious characteristic of an acid: its taste. But acidic foods also stimulate salivation to dilute the acid, which brings out another property of acids—their harsh, even corrosive, nature.
We take advantage of this latter property in everyday life in many ways. Vinegar dissolves the calcium deposits that can form in pots and sinks, and some of today’s cleaning products partially owe their cleansing qualities to the acids they contain. The corrosive nature of acids is also demonstrated by the well- known experiment of soaking a piece of meat or a coin in a cola-based beverage. After several days the meat will have dissolved totally and the surface of the coin will be scarred and pitted.
WHAT IS AN ALKALINE?
Unlike acidic substances, alkaline substances in solution with water give up few or no hydrogen ions. The fewer hydrogen ions they release the less acidic they are or, in other words, the more alkaline.
Also unlike acids, alkaline elements have no corrosive properties. They are gentle substances. Whereas lemon juice causes a sharp burning sensation if applied to a cut, milk does not. Alkaline substances can counter problems caused by acids. Potato juice, for example, soothes the pains of an acid stomach, and milk in large quantities can be an effective method of neutralizing the corrosiveness of acidic poisons swallowed by accident.
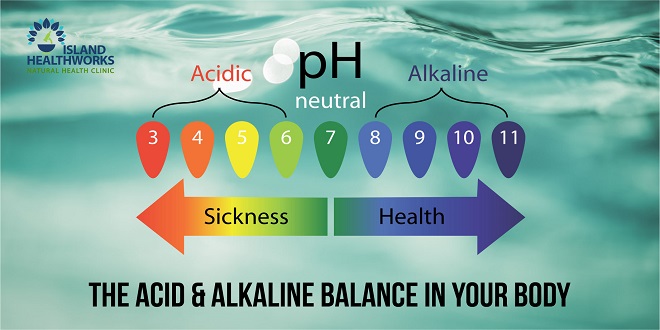
HOW ACIDITY IS MEASURED
As the difference between acids and alkalines is based more or less on their ability to free hydrogen ions, the unit that measures the degree of acidity or alkalinity of a substance is shorthand for the substance’s potential (p) for freeing hydrogen (H) ions, or pH.
DIFFERENCE BETWEEN STRONG AND WEAK ACIDS
In addition to the degree of acidity as measured by the pH scale, acids can be characterized as either strong or weak. In fact, acids are rarely encountered in a free or isolated state; they are most often combined with an alkaline element. When the alkaline combined with the acid is strong (chemically speaking), the acid is of little consequence in the combination. The acid is called weak, as it is easy for the body to reject it. When the alkaline element is weak, however, the acid content is of much greater consequence. It is stable and mixes poorly with other elements, and it is referred to as strong.